|
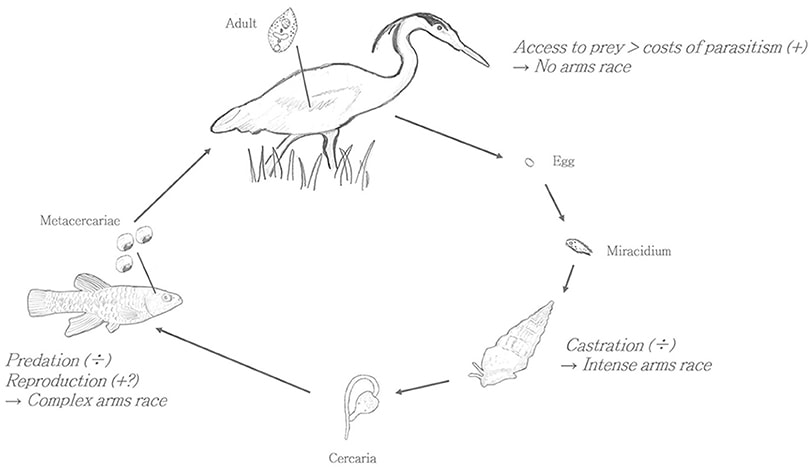
It’s time to lift the veil and take a peek at the “man behind the curtain.” Within the realm of biology, that could mean a lot of things, but in this instance, I’m referring to parasites. Am I comparing the great and powerful Wizard of Oz to a parasite? Just bear with me and this will all make sense by the end of the post. Up to this point in the blog I’ve kind of danced around the margins of this hidden world; there’s some fun discussion of “feathered tree ticks” and kleptoparasites in the post from last March, and I devoted an entire post to brood parasites a few months ago. But parasites and pathogens (which I’ll just combine into “parasites” for simplicity) play an enormous, if underappreciated and often inconspicuous, role in the natural world. And that’s not simply the case because of the SARS-CoV-2 (coronavirus) pandemic. Parasites impact the length and structure of food chains, they can control host populations (by keeping them from growing too large), they galvanized the evolution of one of the most complex physiological systems in nature (the vertebrate immune system), and parasites may be responsible for the evolution of elaborate sexual displays (think of a scarlet tanager’s bright red plumage), as well as the evolution of sexual reproduction. Oh, and they likely make up more than half of the species on our planet. So yeah, (as my wife, the disease ecologist, frequently reminds me) they’re kind of a big deal. One of the first tasks for studying parasites in nature is defining what exactly a parasite is. To do this, we can use some ecological criteria. First, a parasite is an organism that is in an intimate (close proximity) and durable (long-term) relationship with another organism (insert marriage jokes here). That is, they are symbionts. But there are many organisms in intimate and durable relationships with other organisms that are not considered parasitic. Corals and their algal symbionts, and anemones and their attending anemonefish (Nemo) are in intimate and durable relationships, but each has a positive effect on the other. Therefore, they are considered mutualists. A parasite exists in an intimate and durable relationship with another organism upon which it exerts a negative effect. What does a negative effect mean in ecology? Essentially, it means that the parasite creates some sort of a cost for the host, usually in the form of energy loss and often manifesting as reduced reproduction, disease, and in some cases, death. The impact of the parasite isn’t always huge, but the net effect needs to be negative. Is a lion eating a gazelle a parasite? Well, it’s intimate and it’s definitely negative, but it’s not durable. The lion kills the gazelle and uses its resources, but the interaction is short-lived and the act of energy transfer is relatively quick. This interaction is, of course, known as predation. What about a mosquito drawing a blood meal from a person? Are they ‘parasitizing’ us? They are stealing our resources, it’s pretty intimate (the proboscis is literally penetrating our skin), but it’s still not durable. They are considered to be ‘micro-predators’ on us. Ticks, on the other hand, attach to the host for a few days while procuring their blood meal, which is generally long enough to bump them into the true parasite category. Ticks fall under the group of parasites known as ectoparasites, meaning “outside parasites,” and ectoparasites are any parasitic organisms found on the external parts of their host. In addition to ticks, this group includes some very popular members such as lice, fleas, and mites, to name a few. These parasites usually draw their energy from small blood meals, but they can also consume various parts of the host’s integument (i.e. skin, hair, feathers, scales). Feather lice, for example, consume parts of a bird’s feathers, but the award for most bizarre and extreme form of ectoparasitism has to go to Cymothoa exigua: the tongue-eating isopod. Yep, there’s a parasite that attaches to the host’s tongue, and slowly consumes it. Except, it doesn’t just consume the host’s tongue (and don’t worry—the host is always a fish. So far…); it replaces the tongue. With itself. This may be more information that you ever wanted to know about fish and their tongue-stealing parasites, but it’s extraordinary. By assuming the functional role of the tongue, the parasitic isopod allows the fish to continue eating normally (more or less), thereby ensuring that its home and source of food (the fish) remains relatively healthy. The other major group of parasites are the endoparasites, or the “inside parasites.” This category includes everything from intestinal worms like tapeworms, to intracellular protists like malaria. Typically, it’s the intracellular parasites that are the most problematic for the host, and are the hardest for the immune system to kill (hiding inside the cells of the host is a pretty good way to avoid detection). Within the world of parasitology, one of the big questions is: how does the parasite infect a new host? There are few primary ways in which parasites can get to a new host: direct transmission (an infected host touching an uninfected host and transferring the parasite that way); indirect transmission (an infected host contaminating the environment with infective agents that another host can pick-up from the environment); vectored transmission (a biting fly or other biting arthropod carries the parasite from an infected host to an uninfected host); and trophic transmission. And this final form is the one I want to spend some time with. At its most basic level, trophic transmission simply means that the parasite travels from one host to another by being consumed. Seems pretty straight forward, but let’s take a look at a few examples to get better acquainted. The first example comes from California, but this sort of scenario exists all over the world. The parasite in question is a flatworm; a type of trematode called Euhaplorchis californiensis. The parasite’s lifecycle includes three types of host; a snail is the first host, a fish is the intermediate host, and birds are the definitive host. Ultimately, the parasite wants to wind up in a bird, where it hopes to meet others of its kind and begin reproducing (the bird’s gut is like a singles bar for these worms). But how does the parasite get from a snail to this feathered singles bar? First, an infected bird poops eggs of the trematode into the salt-water marsh habitat that the snail lives in. The eggs are accidentally consumed by the snail as it grazes on algae (or in some cases the egg hatches in the environment and the snail is infected by a swimming miracidium), and once inside the snail, the next stage of the parasite begins developing in the snail’s gonads. Here, in this large gonadal space, the parasite consumes the host’s reproductive tissue (testes and ovaries) and converts that reproductive matter into the parasite’s next infective stage: the cercariae. The cercariae are released into the water, and these free-swimming, sperm-resembling parasites seek out the next host: a California killifish. Once they’ve found a killifish, they burrow into the tissue, lose their tail, and head for the fish’s head. The parasites are actually looking to encyst on the fish’s brain, where they turn into metacercariae. Recall that the parasite would very much like to get into the stomach of a bird so it can finish its development and begin sexually reproducing. But the fish has no interest whatsoever in going on this bird-stomach road-trip. Given that the parasite is inside the fish, this would seem like a pretty substantial hurdle for the parasite to overcome. And it is here that the parasite begins pulling its puppet strings. Killifish that are infected with the trematode metacercariae start to act a little differently than their school-mates. They exhibit a suite of behaviors that would seem to be at odds with their goal of blending in with the crowd. They begin swimming closer to the surface of the water, and do more darts, twists and turns than their neighbors. All of these erratic, conspicuous behaviors increase the visibility of the bizarre-acting fish (think Alfredo Linguini being controlled by Remy in the movie Ratatouille) and serve to catch the attention of fish-eating birds in the vicinity. Research by scientists at the University of California, Santa Barbara has shown that infected fish are 10-30 times more likely to be eaten by a bird than uninfected fish. And once inside the bird’s gut, the parasites can again find mates, reproduce, and lay eggs that are dropped into the salt marsh, thus completing the Euhaplorchis circle of life. But let’s talk a bit more about the cysts on the fish’s brain, and that moment when a large bird comes along and eats a weird-behaving and infected fish. Well, the parasite seems to have gotten its wish, but is this purely a product of an infected fish acting weird because it has a bunch of cysts on its brain (which would be understandable), or is the parasite actually manipulating the behavior of the host in specific ways that increase the probability of getting eaten by a bird? The evidence seems to support the latter. Infected fish have different neurochemical profiles than uninfected fish, specifically with respect to serotonin and dopamine. Both of these hormones are linked to swimming behavior and potentially anti-predator behavior, and by reducing serotonin and increasing dopamine, the parasite may be forcing the fish to behave in ways that increase its chances of getting munched. And that’s how you execute the old “snail to fish to bird” maneuver. What’s more incredible to me than this instance of host behavior modification, is that there are hundreds, and perhaps thousands of similar, but distinct examples of host manipulation by parasites. Another trophically transmitted parasite is Toxoplasma gondii, which causes toxoplasmosis in humans. The definitive hosts (where the parasite reproduces sexually) for T. gondii are cats; house cats, bobcats, mountain lions, African lions, etc. The intermediate host (and in this system, there’s generally just a first intermediate host and the definitive host) is typically a rodent, although there’s some intriguing evidence to indicate that other animals like raccoons, baboons, and even humans may act as intermediate hosts. We were, after all, lower down on the food chain for many, many years, and probably served as a not-too infrequent source of food for big cats like leopards, lions, and tigers. In the more typical rodent-cat scenario, the rodent gets infected by ingesting T. gondii cysts from the environment. The challenge for the parasite is now getting from its rodent host to the thing that the rodent host is most likely to avoid; a cat. No problem. All the parasite has to do is convince the rodent that the aroma of cat (especially cat urine) is magnetically alluring, and demands further investigation. Indeed, the parasite has somehow managed to pull off this feat (we aren’t sure how the parasite manipulates the hosts’ behavior in this case), and even makes the rodent more likely to approach any cats that happen to be in the area. Bad for rodent, good for parasite. Once the rodent is consumed, the parasite can mature and begin the sexual reproduction phase of its life in the gut of the cat. The complete T. gondii story is much more complicated and involves fascinating journeys into human behavior; for one, is the “crazy cat lady” actually infected with T. gondii and exhibiting behavior that might have, at one time, increased the chances of her getting eaten by a leopard? It’s certainly possible, although a bit tricky to test. People can also get infected by eating raw or undercooked meat (prevalence of toxoplasmosis in France, where they really enjoy their steak tartare, used to be upwards of 80% of the population, although it appears to be closer to 50% now), and apparently rodents (and perhaps humans) can transmit the parasite via sexual contact. Not all behavior manipulation is linked to trophically transmitted parasites. Probably the best known example, and now famous from the Girl with all the Gifts book/movie, is the “zombie-fungus” Ophiocordyceps. This genus is composed of over two hundred different fungi that infect insects and spiders, and hijack their bodies. Unlike the Euhaplorchis-killifish system wherein the parasite seems to be controlling the host by manipulating the brain, the fungus appears to use biochemical compounds to interfere with the host’s nervous system, and then takes direct control of the musculature. In an ant host, the fungus forces the ant to seek out a humid spot (good for fungal development) where the ant will clamp onto the vegetation with its jaws. The ant eventually dies, and a few days later, the fungus bursts from the dead ant, sending out fruiting bodies (spores) that will be scattered by the wind to infect a new host. If you’ve made it this far without blanching at the subject matter, congratulations! I know it can be uncomfortable to confront this amazing and horrifying world in which seemingly normal organisms are being controlled by hidden agents. And while this may all sound like a bad dream, I can assure you, we’re very much still in Kansas.
Next post: The Other Side of the Coin: Host Immuno-ecology Subscribe to the Newsletter! If you would like to get notifications about when new posts are up and other tidbits related to the blog, sign up for the View Out the Door twice-monthly newsletter. Just email viewoutthedoor “at” gmail “dot” com with the subject header SUBSCRIBE. And if you’d like to unsubscribe, e-mail with the subject header UNSUBSCRIBE.
1 Comment
2/2/2021 11:09:25 am
When it comes to your health, trust Harbor Compounding Pharmacy to come up with innovative and customized solutions for you.
Reply
Leave a Reply. |
About the author:Loren grew up in the wilds of Boston, Massachusetts, and honed his natural history skills in the urban backyard. He attended Cornell University for his undergraduate degree in Natural Resources, and received his PhD in Ecology from the University of California, Santa Barbara. He has traveled extensively, and in the past few years has developed an affliction for wildlife photography. Archives:
|