|
Most temperate regions of the world abide by the classical four season paradigm: Winter, Spring, Summer, Fall. Maine, with its strong independent streak has nine. Maybe more. There are, of course, the classical seasons, but Maine offers up a few more, very specific, mini-seasons. Unlike the big four, these mini-seasons don’t have calendar start and end dates. Instead, they are defined by meteorological or biological phenomena. For example, between Winter and Spring is Mud Season. Mud Season is the period during which the ground begins to melt, but before the vegetation has begun growing. The ground thaw typically starts at the surface and progresses downwards, which means that the moisture from the melting soil can get trapped between the top layer and the retreating ice. Extra-moist soil happens to be the key ingredient for mud, and voila! Mud Season. For most Mainers, Mud Season is something to be tolerated as they wait for the emergence of spring, and the return of nice weather. But there are some for whom Mud Season represents a return to simpler times. It presents an opportunity for these people to put on their galoshes and go jump in a mud puddle. Of course, for many people, galoshes have been replaced by oversized trucks, and mud puddles have been replaced by just about anything these folks can drive their oversized trucks through. A little bit later in the year lies Maine’s most sinister season: Black Fly Season. This season actually reminds me a bit of one of those deep-sea predatory fishes, the anglerfish. For those not familiar with this denizen of the dark, anglerfish have a unique anatomical feature for which they are named; they sport a modified dorsal spine that protrudes out in front of their body, and at the end of this spine sits a bioluminescent “lure” that the anglerfish dangles in front of a mouth overflowing with teeth. The luminous lure attracts other inhabitants of the deep who think they’ve found a tasty glowing meal, only to realize too late that they are that meal. Black Fly Season is just like that anglerfish. Let me explain. People in Maine endure a rather extended Winter, which is then followed by Mud Season. By the time warm weather arrives, many folks are chomping at the bit to get outside, enjoy outdoor activities, and soak up some vitamin D. So, with the flowers blooming and the trees freshly attired in the greens they just pulled out of winter storage, people head out into the woods in droves. And that’s when the anglerfish-black flies spring their trap. These tiny, blood-feeding micro-predators seem to regard insect repellent as a dinner bell, and long-sleeves/long-pants and head-nets as dining room decor. Unlike the gangly mosquito, which is all legs and proboscis, the black fly is a compact little rover. They crawl up your pant legs, down your collar, under the head-net perimeter, and anywhere else (yes, anywhere else) they can smell that sweet CO2 elixir oozing out. And the bite of a black fly is an order of magnitude worse than that of a mosquito. For one thing, rather than inserting a hypodermic needle, they lacerate your skin and lap up the pooling blood. Once the biting begins, so does the psychosis. A few Junes back, Tara and I made the questionable decision to camp at Baxter State Park, deep in Maine’s north-central woods, during one of the worst Black Fly Seasons in recent history (according to the locals). Of course, we didn’t know this when we decided to head up there, and in fact, we thought the season had already passed. We were wrong. When we arrived at the park, we were full of excitement and anticipation. There were moose to see, mountains to hike, sunsets to photograph…we were mesmerized by that big glowing lure. And then the teeth closed around us. We realized we were in trouble pretty quickly. Like within five minutes of getting out of the car. The first squadron made contact while we were setting up our tent, and for the next three days our bodies were under siege. We wore long sleeves and long pants. We donned jackets. We tucked our pants into our socks. We put on head-nets. We bathed in bug spray. None of it mattered. I would sit on the shores of Sandy Stream Pond, trying to capture images of the surrounding mountains with my camera, and the black flies would crawl down the back of my pants, or find the seams in my socks where the fabric was thinnest. We climbed Mt. Katahdin, the second highest peak in New England, hoping we would lose them during the ascent, but they followed us almost to the summit. We ate meals in our car when we were at camp, and they squirreled their way inside for a quick bite of their own. We retreated into our tent, but they were there too, waiting for us among the folds of our sleeping bags. Nowhere was safe. The bites, full of the black fly’s special cocktail of numbing agent and anticoagulant, itched incessantly, and extracted a hefty mental toll on us. But it was the fear of more bites to come that finally drove us out. If I never experience another Black Fly Season, it will be far too soon. Moving from blood-meals to Maine-meals, Maine boasts a few other, culinary-oriented, seasons that are each deserving of attention, but which I’ll mention only briefly here: Maple Syrup Season (also called Sugaring Season), Blueberry Season, and the now-defunct Shrimp Season. But the undisputed king of the seasons for most Mainers and visitors (those “from away”) is Summer. For every one of my 43 years, I’ve spent at least some portion of the summer in Maine. When I was young (in the 0-9 years range), that slice consisted of one golden week in August when we would make the four-hour drive from our house in Boston to a family enclave of sorts in McFarland’s Cove. I say that it was a four-hour drive, and technically if you think of time as a constant, linear phenomenon, it did take four hours. But to a five-year old boy who was practically apoplectic with excitement, the drive involved multiple time loops, some serious backwards time travel, and actually took two weeks. As we turned off the country highway onto McFarland’s Cove Road, however, the seeimingly interminable trip was forgotten. McFarland’s Cove is one of an almost infinite number of inlets tucked into Maine’s rocky Mid-coast region. Maine’s coast, which looks like it was drawn by a hyperactive four-year-old on a sugar high, has almost 3,500 miles of coastline crammed into 228 straight-line miles. Imagine the output from a seismograph during an earthquake, and you’ve got a good idea of what the coastline looks like. For my younger self, that one cove was the center of my universe. I would pack about a month’s worth of swimming, fishing, rock-flipping and rock-skipping, snorkeling, tide-pooling, crabbing, snake-hunting, and general exploring into that one decadent week. A typical day would begin with an individual cereal box breakfast (if I was lucky, it would be some sugary delight like Froot Loops, or Lucky Charms), and once breakfast was done, I was free to roam in my natural habitat. At that time, my family lived in an urban Boston neighborhood, and while we had a lovely big backyard, for me it felt like being a little two-legged tiger confined to a zoo exhibit. I dreamt of days spent outside in pursuit of animals, and once I was in Maine, those dreams became my reality. Some days I would spend 8 hours swimming around in the chilly Atlantic water, mask and snorkel gradually adhering to my face as I searched for marine life. And the cove was full of it; crabs and hermit crabs, skates and flounders, sea urchins and sea stars, the eel-like rock gunnel, and the always thrilling lobster. Whenever I would dive down among the large patches of brown algae, however, my heart would quicken at the thought of what might be hidden among the thick fronds: the grotesque, and sure to swallow-me-whole, goosefish. There are, of course, more dangerous animals in the ocean than goosefish, and while I don’t think they keep stats on worldwide goosefish attacks, I’m pretty positive no one has ever been eaten by one. But I can pinpoint the moment the goosefish became lodged in my psyche. I was going through a field guide to the plants and animals of the eastern US when I came across its image. The goosefish, which happens to be a type of anglerfish like our deep-sea friend described above, is a bottom-dwelling predator that looks as though it has been stepped on and then covered in bits of brown doilies to conceal the damage. But the thing that captivated and terrified me was its head, which comprises about half the fish’s body and is full of dagger-like teeth. When you consider that the goosefish can approach lengths of five feet, you begin to see why it was the risk of goosefish attack that kept me from exploring the darker recesses of the cove. There may not have been records of goosefish-related deaths at that time, but I certainly wasn’t going to become the first. Here’s a fun video of a goosefish in action so you can appreciate the fear I experienced (start at 55 seconds). Despite the constant threat to life and limb, there was no place I would rather spend time. Eventually, though, I would get hungry, tired, or numb, and would have to retreat to the land. The terrestrial environs of the cove presented their own suite of exciting animals to pursue, but there was one animal that lay on a pedestal above all others: the garter snake. I loved snakes and had an insatiable appetite for catching them. I was never satisfied by simply finding one; I had to have it in my hands. Only then, when it was slithering through my fingers, biting me with its tiny teeth, and dousing me with its rancid musk was I happy. And I wanted those around me to be happy too, so I tried sharing my snake captures with others. The notion that everyone wouldn’t want a snake in their hands, or better yet, slithering down their shirt, was outside my purview at first. I was quickly disabused of my snake-outreach ideas, however, and had to content myself with sharing my snake passion with just a few other like-minded boys in the cove.
Over time, I grew out of the need to catch and hold things that I found, but it took a while. I suspect my passion for photography is an evolution of sorts for this sort of behavior. It provides an opportunity for me to capture and hold the animal without touching it. And I don’t smell of snake stink when I’m done. Next post: Summers in Maine: Part II. Subscribe to the Newsletter! If you would like to get notifications about when new posts are up and other tidbits related to the blog, sign up for the View Out the Door twice-monthly newsletter. Just email viewoutthedoor “at” gmail “dot” com with the subject header SUBSCRIBE. And if you’d like to unsubscribe, e-mail with the subject header UNSUBSCRIBE.
2 Comments
The wolf materialized so silently from among the ponderosa pines, it was as though the trees had simply breathed it into existence. Everywhere around me within the Yellowstone Caldera, the land was exhaling- hydrothermal vents steamed, hot springs simmered, mud pools bubbled, and geysers erupted- but when I spotted the large canine trotting out into the sagebrush just 25 feet away from me, my breathing paused for a second. This was a moment I had been waiting years for; a close encounter with a wolf in the wild. And while wolves are one of Yellowstone’s big attractions these days, a little over a quarter century ago, there were no resident wolves in the park. Historically, wolves occupied the vast majority of North America, with an estimated 2 million animals roaming from northern Canada and Alaska down into Mexico. But an aggressive (and federally funded) eradication campaign resulted in the extirpation of wolves from all of the lower 48 states, with the exception of small populations in Michigan and Minnesota, as well as a few individuals of the Mexican gray wolf subspecies in Arizona/New Mexico, and a few of the red wolf subspecies/species in Louisiana/Texas. In the 1960s, federal protection was granted for wolves in the lower 48, and in January 1995, 8 wolves from Jasper National Park, in Alberta, Canada were trucked down to Yellowstone National Park and released. These wolves were the first to set foot in the park since the 1920s, and over the next two years, they were joined by an additional 33 animals. During the next two and a half decades, the Greater Yellowstone ecosystem experienced some dramatic changes as the wolves established packs throughout the park and expanded into the surrounding areas. The return of the wolf had a very immediate and direct impact on two species that had become quite abundant during the wolf’s absence- elk and coyotes- and indirect effects on hundreds of other species. Wolves are both top predators and keystone species, and their return set-off a cascade of ecological events (called a “trophic cascade”), with ripple effects spreading to almost every habitat and community in the region. In the absence of wolves, elk numbers exploded, with upwards of 20,000 elk wandering inside the park’s boundaries. Wolves are the primary predator of elk, and without wolves to keep their population in check, elk levels far exceeded the area’s carrying capacity. This meant that during harsh winters, or periods of drought, thousands of elk could starve to death. Now, 25 years after the wolf reintroduction, elk numbers have stabilized at between 6,000 and 8,000 animals. Researchers who have been studying the ecological effects of the wolf reintroduction say elk no longer starve to death in appreciable numbers during the winter. Moreover, by keeping the elk population down and selectively killing individuals in poorer condition, the wolves may be creating elk herds that are more resilient to environmental variability. This could help elk populations cope with increased climatic volatility associated with climate change. Researchers also found that wolves changed their hunting behavior in response to environmental conditions. In years with normal amounts of precipitation, wolves target older female elk, which are the easiest to hunt. But during dryer years when forage is lower quality, wolves take a much higher percentage of bulls. Bull elk eat relatively little during the mating period, or “rut”, when they expend enormous amounts of energy battling for dominance. In years with reduced precipitation and poor-quality food, bulls can be especially vulnerable to predation. The impact of wolves on coyotes is no less striking than their effect on elk, but it occurred via competition rather than predation. Wolves are the primary competitors of coyotes, and with their larger, more dominant cousins out of the way, coyote numbers shot up in the park. While coyotes are unable to take down an adult elk, they fed on deer and the calves of many of the park’s ungulates. They also took advantage of the high number of winter-killed elk. When wolves returned, coyote numbers in some areas of the park declined by 50%. Wolves will kill coyotes in the same manner that lions seek out and try to kill leopards and cheetahs; eliminate the competition, and there will be more prey for you. Most of the coyotes that are killed by wolves these days are those that are not patient or wary enough at a kill site; a coyote that comes in to a wolf kill while the wolves are still feeding is unlikely to survive to the next breeding season. But what about the indirect effects that wolves have had on so many other species in the park? The high number of elk present before the wolf return resulted in overgrazing of many areas, especially the riparian zones bordering streams and rivers. Not only were there lots of hungry elk, but the elk would settle in an area and eat everything available. The loss or reduction of willow, aspen, cottonwood, and berry-producing shrubs from these habitats also negatively impacted bird species that use those trees and shrubs for nesting and food, as well as beaver, which depend on willows in the winter months. Beavers are themselves important ecological players, and their disappearance from the landscape resulted in largescale changes. Beavers are called “ecological engineers” because their damns create ponds and wetlands and alter the hydrology of streams and rivers. The habitats they create are important for a wide array of animals, from aquatic invertebrates, fish, and amphibians, to waterfowl and moose. The return of wolves has had a positive impact on the ecosystem in two ways: they’ve reduced the number of elk overall, and the elk that remain no longer settle in riparian zones and remove all the edible vegetation. The reason for the latter is that wolves often travel along streams and rivers, thereby providing a reasonably strong deterrent to the elk. This wolf-driven alteration in the elks’ behavior, and the landscape-level effects associated with it, is now referred to as a “landscape of fear.” Essentially, this means that in places where predators are present, their prey have to adjust their behavior to reduce the risk of predation. Usually this entails spending more time watching for predators (at the expense of time spent eating), and avoiding areas where the predator is likely to occur or where the prey cannot see the predator coming. Today, the Greater Yellowstone ecosystem much more closely resembles a healthy, intact ecosystem than it did prior to wolf reintroduction. Wolf numbers within the park rise and fall from year to year, but over the past decade have mostly fluctuated between 83 and 108 wolves. This past year there were about 95 wolves spread across eight packs. These packs are heavily monitored by wildlife biologists, and there is very high-resolution information about the genealogy of just about every individual in the park. In addition, biologists know much of the social structure of each pack, as well as the soap opera levels of drama that can occur. For example, in 2019, a subordinate female of the Junction Butte pack (found in the northern portion of the park) killed the pups of the alpha female, and the pack then raised the subordinate female’s pups. And in 2000, a group of subordinate females from the Druid pack (no longer in existence), formed an alliance, and in true coup d’etat fashion, killed the pack’s alpha female. Despite the death of the alpha female, the pack continued to raise her pups, albeit in a new den. The wolves of Yellowstone have acquired a bit of celebrity status, and people follow the trials and tribulations of the wolves in online forums, and at the park. When I was there in late September into early October, Lamar Valley was a hotbed of wolf-watching activity. Each morning and evening, when the wolves were more active, people would be lined up along the road by the hundreds. The focus of their attention was the Junction Butte pack, which had obligingly set-up on the other side of the valley. Most of the park’s wolf packs number between eight and 10 individuals, but the Junction Butte pack had 35 this year, 18 of which were born in 2020. People would bring lawn chairs, spotting scopes, and binoculars to watch the pack’s activity, and swap stories of the latest wolf gossip. One of the first things people notice about wolves within Yellowstone is the large number of wolves with black fur; in fact, almost 50% of the wolves are black. This color morph is linked to a single gene thought to have originated in domestic dogs from the Old World. North American wolves may have acquired this gene by hybridizing with domestic dogs brought over by people crossing the Bering Land Bridge within the last 7,000 years. Melanization (i.e. dark coloring) is linked to immune function in many species, from insects to mammals, and it appears that dark-colored wolves have better immune defenses against canine distemper (caused by a paramyxovirus), and may be more resistant to mange (caused by mites) than grey-colored wolves. Severe outbreaks of canine distemper (in 1995, 2005, and 2008) as well as mange (2007) may have selected for black-coated wolves within the park, and even throughout the Rockies. So why aren’t all the wolves in the park black now? It turns out that grey wolves tend to be more dominant during aggressive interactions, and grey-colored females tend to have larger litters than females with black fur. Grey individuals thus have greater fecundity (more offspring) when parasite and pathogen pressure are low, and black wolves have higher pup survival during disease outbreaks. In addition to the black and gray coloration, there are a few white wolves in the park. One of these is the alpha female of the Wapiti Lake wolf pack, and a few minutes before my interaction with the grey wolf described in the opening paragraph, I had come across this beautiful white wolf. My initial glimpse of her was at some distance, when I saw her padding along the edge of a large stream. I headed off in her direction, hoping to get some shots that would show something more than a dog-shaped dot on the horizon. I quickly lost track of her, however, due to the undulating nature of the terrain, and stopped to consider my options. I was about to head back towards my car, when I caught movement out of the corner of my eye. On a nearby wooded hill, a white shape was gliding among the trees. When she caught site of me, she paused, and after surveying the area, she loped off, disappearing over the hill’s ridge. I hurried back to the road, hoping to catch a glimpse of her as she entered the broad expanse of sagebrush, but I couldn’t see her anywhere. I looked out into the meadow and saw a massive black wolf trotting through the grass. He was joined by a much smaller grey wolf, and then the white wolf appeared, making for the black and grey members of the pack. It was at this moment that the grey wolf emerged from the pines just to my right, and began his foray out into the sagebrush. Instead of going directly to the other members of the pack, he instead turned part ways towards me and began howling. Hearing a wolf howl in the wild is a transcendent experience. For me, it encapsulates the soul of the wilderness. And having a wolf howl in such close proximity… well, it was indescribably powerful. And now, thanks to passage of a ballot initiative in the November 2020 election calling for the reintroduction of wolves in Colorado, this is an experience that people in the Colorado Rockies may be able to experience within a few years. The wolf, like so much of our world, has become politicized and polarized, and this was on full display leading up to the election. A deep-seated dislike of predators still permeates a large section of the population, especially among hunters and livestock owners. Hunters don’t like the competition, and livestock owners don’t like the risk to their livelihood. And both have valid concerns; elk numbers have dipped in places where wolves returned, and wolves do kill livestock. But perception and reality are not always linked. Wolves may decrease the number of elk, but wolves generally target older and weaker individuals, whereas hunters prefer healthy adults in their prime. Colorado has an estimated 287,000 elk, and wolf reintroduction could have a positive impact on the health of the elk population, similar to what has happened in Yellowstone. In addition, there likely will be ripple effects which may benefit hunters in the long term. Decreases in coyote numbers may lead to increased deer fawn survival, and reduced levels of overgrazing by elk and moose could benefit game birds like grouse and turkeys. With regards to livestock, verified depredation by wolves accounts for a fraction of a percent of livestock mortality, even across the northern Rocky Mountain range where wolves now live. By far the greatest causes of mortality are health and weather-related deaths. Does wolf-predation still affect the bottom line for livestock managers? For some, yes. Are there ways to mitigate this cost? Absolutely. This is part of what researchers and state officials will be working on as they develop a Colorado wolf reintroduction plan over the next three years. The wolves of Yellowstone have taught us an incredible amount about the importance of top predators to the health of an ecosystem. Now we just have to find ways to coexist with them. Next post: TBD.
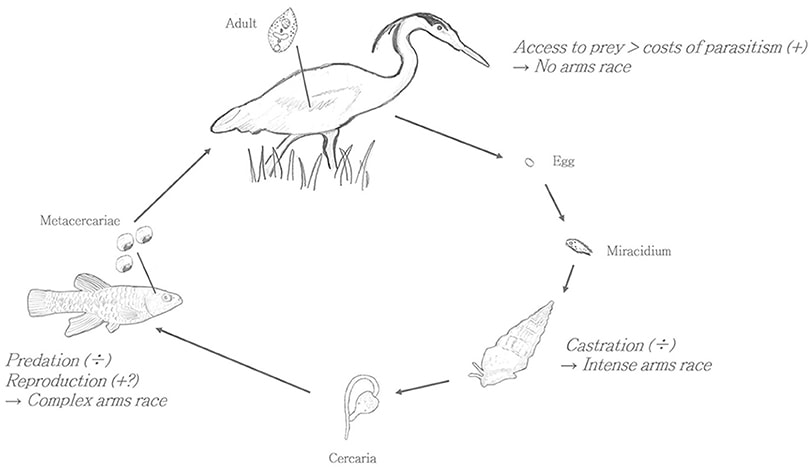
Subscribe to the Newsletter! If you would like to get notifications about when new posts are up and other tidbits related to the blog, sign up for the View Out the Door twice-monthly newsletter. Just email viewoutthedoor “at” gmail “dot” com with the subject header SUBSCRIBE. And if you’d like to unsubscribe, e-mail with the subject header UNSUBSCRIBE. It’s time to lift the veil and take a peek at the “man behind the curtain.” Within the realm of biology, that could mean a lot of things, but in this instance, I’m referring to parasites. Am I comparing the great and powerful Wizard of Oz to a parasite? Just bear with me and this will all make sense by the end of the post. Up to this point in the blog I’ve kind of danced around the margins of this hidden world; there’s some fun discussion of “feathered tree ticks” and kleptoparasites in the post from last March, and I devoted an entire post to brood parasites a few months ago. But parasites and pathogens (which I’ll just combine into “parasites” for simplicity) play an enormous, if underappreciated and often inconspicuous, role in the natural world. And that’s not simply the case because of the SARS-CoV-2 (coronavirus) pandemic. Parasites impact the length and structure of food chains, they can control host populations (by keeping them from growing too large), they galvanized the evolution of one of the most complex physiological systems in nature (the vertebrate immune system), and parasites may be responsible for the evolution of elaborate sexual displays (think of a scarlet tanager’s bright red plumage), as well as the evolution of sexual reproduction. Oh, and they likely make up more than half of the species on our planet. So yeah, (as my wife, the disease ecologist, frequently reminds me) they’re kind of a big deal. One of the first tasks for studying parasites in nature is defining what exactly a parasite is. To do this, we can use some ecological criteria. First, a parasite is an organism that is in an intimate (close proximity) and durable (long-term) relationship with another organism (insert marriage jokes here). That is, they are symbionts. But there are many organisms in intimate and durable relationships with other organisms that are not considered parasitic. Corals and their algal symbionts, and anemones and their attending anemonefish (Nemo) are in intimate and durable relationships, but each has a positive effect on the other. Therefore, they are considered mutualists. A parasite exists in an intimate and durable relationship with another organism upon which it exerts a negative effect. What does a negative effect mean in ecology? Essentially, it means that the parasite creates some sort of a cost for the host, usually in the form of energy loss and often manifesting as reduced reproduction, disease, and in some cases, death. The impact of the parasite isn’t always huge, but the net effect needs to be negative. Is a lion eating a gazelle a parasite? Well, it’s intimate and it’s definitely negative, but it’s not durable. The lion kills the gazelle and uses its resources, but the interaction is short-lived and the act of energy transfer is relatively quick. This interaction is, of course, known as predation. What about a mosquito drawing a blood meal from a person? Are they ‘parasitizing’ us? They are stealing our resources, it’s pretty intimate (the proboscis is literally penetrating our skin), but it’s still not durable. They are considered to be ‘micro-predators’ on us. Ticks, on the other hand, attach to the host for a few days while procuring their blood meal, which is generally long enough to bump them into the true parasite category. Ticks fall under the group of parasites known as ectoparasites, meaning “outside parasites,” and ectoparasites are any parasitic organisms found on the external parts of their host. In addition to ticks, this group includes some very popular members such as lice, fleas, and mites, to name a few. These parasites usually draw their energy from small blood meals, but they can also consume various parts of the host’s integument (i.e. skin, hair, feathers, scales). Feather lice, for example, consume parts of a bird’s feathers, but the award for most bizarre and extreme form of ectoparasitism has to go to Cymothoa exigua: the tongue-eating isopod. Yep, there’s a parasite that attaches to the host’s tongue, and slowly consumes it. Except, it doesn’t just consume the host’s tongue (and don’t worry—the host is always a fish. So far…); it replaces the tongue. With itself. This may be more information that you ever wanted to know about fish and their tongue-stealing parasites, but it’s extraordinary. By assuming the functional role of the tongue, the parasitic isopod allows the fish to continue eating normally (more or less), thereby ensuring that its home and source of food (the fish) remains relatively healthy. The other major group of parasites are the endoparasites, or the “inside parasites.” This category includes everything from intestinal worms like tapeworms, to intracellular protists like malaria. Typically, it’s the intracellular parasites that are the most problematic for the host, and are the hardest for the immune system to kill (hiding inside the cells of the host is a pretty good way to avoid detection). Within the world of parasitology, one of the big questions is: how does the parasite infect a new host? There are few primary ways in which parasites can get to a new host: direct transmission (an infected host touching an uninfected host and transferring the parasite that way); indirect transmission (an infected host contaminating the environment with infective agents that another host can pick-up from the environment); vectored transmission (a biting fly or other biting arthropod carries the parasite from an infected host to an uninfected host); and trophic transmission. And this final form is the one I want to spend some time with. At its most basic level, trophic transmission simply means that the parasite travels from one host to another by being consumed. Seems pretty straight forward, but let’s take a look at a few examples to get better acquainted. The first example comes from California, but this sort of scenario exists all over the world. The parasite in question is a flatworm; a type of trematode called Euhaplorchis californiensis. The parasite’s lifecycle includes three types of host; a snail is the first host, a fish is the intermediate host, and birds are the definitive host. Ultimately, the parasite wants to wind up in a bird, where it hopes to meet others of its kind and begin reproducing (the bird’s gut is like a singles bar for these worms). But how does the parasite get from a snail to this feathered singles bar? First, an infected bird poops eggs of the trematode into the salt-water marsh habitat that the snail lives in. The eggs are accidentally consumed by the snail as it grazes on algae (or in some cases the egg hatches in the environment and the snail is infected by a swimming miracidium), and once inside the snail, the next stage of the parasite begins developing in the snail’s gonads. Here, in this large gonadal space, the parasite consumes the host’s reproductive tissue (testes and ovaries) and converts that reproductive matter into the parasite’s next infective stage: the cercariae. The cercariae are released into the water, and these free-swimming, sperm-resembling parasites seek out the next host: a California killifish. Once they’ve found a killifish, they burrow into the tissue, lose their tail, and head for the fish’s head. The parasites are actually looking to encyst on the fish’s brain, where they turn into metacercariae. Recall that the parasite would very much like to get into the stomach of a bird so it can finish its development and begin sexually reproducing. But the fish has no interest whatsoever in going on this bird-stomach road-trip. Given that the parasite is inside the fish, this would seem like a pretty substantial hurdle for the parasite to overcome. And it is here that the parasite begins pulling its puppet strings. Killifish that are infected with the trematode metacercariae start to act a little differently than their school-mates. They exhibit a suite of behaviors that would seem to be at odds with their goal of blending in with the crowd. They begin swimming closer to the surface of the water, and do more darts, twists and turns than their neighbors. All of these erratic, conspicuous behaviors increase the visibility of the bizarre-acting fish (think Alfredo Linguini being controlled by Remy in the movie Ratatouille) and serve to catch the attention of fish-eating birds in the vicinity. Research by scientists at the University of California, Santa Barbara has shown that infected fish are 10-30 times more likely to be eaten by a bird than uninfected fish. And once inside the bird’s gut, the parasites can again find mates, reproduce, and lay eggs that are dropped into the salt marsh, thus completing the Euhaplorchis circle of life. But let’s talk a bit more about the cysts on the fish’s brain, and that moment when a large bird comes along and eats a weird-behaving and infected fish. Well, the parasite seems to have gotten its wish, but is this purely a product of an infected fish acting weird because it has a bunch of cysts on its brain (which would be understandable), or is the parasite actually manipulating the behavior of the host in specific ways that increase the probability of getting eaten by a bird? The evidence seems to support the latter. Infected fish have different neurochemical profiles than uninfected fish, specifically with respect to serotonin and dopamine. Both of these hormones are linked to swimming behavior and potentially anti-predator behavior, and by reducing serotonin and increasing dopamine, the parasite may be forcing the fish to behave in ways that increase its chances of getting munched. And that’s how you execute the old “snail to fish to bird” maneuver. What’s more incredible to me than this instance of host behavior modification, is that there are hundreds, and perhaps thousands of similar, but distinct examples of host manipulation by parasites. Another trophically transmitted parasite is Toxoplasma gondii, which causes toxoplasmosis in humans. The definitive hosts (where the parasite reproduces sexually) for T. gondii are cats; house cats, bobcats, mountain lions, African lions, etc. The intermediate host (and in this system, there’s generally just a first intermediate host and the definitive host) is typically a rodent, although there’s some intriguing evidence to indicate that other animals like raccoons, baboons, and even humans may act as intermediate hosts. We were, after all, lower down on the food chain for many, many years, and probably served as a not-too infrequent source of food for big cats like leopards, lions, and tigers. In the more typical rodent-cat scenario, the rodent gets infected by ingesting T. gondii cysts from the environment. The challenge for the parasite is now getting from its rodent host to the thing that the rodent host is most likely to avoid; a cat. No problem. All the parasite has to do is convince the rodent that the aroma of cat (especially cat urine) is magnetically alluring, and demands further investigation. Indeed, the parasite has somehow managed to pull off this feat (we aren’t sure how the parasite manipulates the hosts’ behavior in this case), and even makes the rodent more likely to approach any cats that happen to be in the area. Bad for rodent, good for parasite. Once the rodent is consumed, the parasite can mature and begin the sexual reproduction phase of its life in the gut of the cat. The complete T. gondii story is much more complicated and involves fascinating journeys into human behavior; for one, is the “crazy cat lady” actually infected with T. gondii and exhibiting behavior that might have, at one time, increased the chances of her getting eaten by a leopard? It’s certainly possible, although a bit tricky to test. People can also get infected by eating raw or undercooked meat (prevalence of toxoplasmosis in France, where they really enjoy their steak tartare, used to be upwards of 80% of the population, although it appears to be closer to 50% now), and apparently rodents (and perhaps humans) can transmit the parasite via sexual contact. Not all behavior manipulation is linked to trophically transmitted parasites. Probably the best known example, and now famous from the Girl with all the Gifts book/movie, is the “zombie-fungus” Ophiocordyceps. This genus is composed of over two hundred different fungi that infect insects and spiders, and hijack their bodies. Unlike the Euhaplorchis-killifish system wherein the parasite seems to be controlling the host by manipulating the brain, the fungus appears to use biochemical compounds to interfere with the host’s nervous system, and then takes direct control of the musculature. In an ant host, the fungus forces the ant to seek out a humid spot (good for fungal development) where the ant will clamp onto the vegetation with its jaws. The ant eventually dies, and a few days later, the fungus bursts from the dead ant, sending out fruiting bodies (spores) that will be scattered by the wind to infect a new host. If you’ve made it this far without blanching at the subject matter, congratulations! I know it can be uncomfortable to confront this amazing and horrifying world in which seemingly normal organisms are being controlled by hidden agents. And while this may all sound like a bad dream, I can assure you, we’re very much still in Kansas.
Next post: The Other Side of the Coin: Host Immuno-ecology Subscribe to the Newsletter! If you would like to get notifications about when new posts are up and other tidbits related to the blog, sign up for the View Out the Door twice-monthly newsletter. Just email viewoutthedoor “at” gmail “dot” com with the subject header SUBSCRIBE. And if you’d like to unsubscribe, e-mail with the subject header UNSUBSCRIBE. There are a few things about birds that everyone knows: they have feathers, most can fly, they lay eggs, and most of them build nests. If I asked you to picture a bird’s nest, you would probably conjure up images of a small nest built of twigs and grass, with a nice bowl-shape to it. This is called an “open-cup” nest and is the quintessential songbird nest. But this is just the tip of the "nest-berg"; there are mud-pedestal nests, Dutch-oven nests, nests made of saliva, hanging nests intricately woven from grasses, tree-cavity nests, and giant mound nests made of rotting vegetation.  A nest mound constructed from rotting vegetation, built by an Australia Brushturkey. These birds are in the family Megapodiidae, and all members of this family construct some sort of mound nest in which they deposit their eggs, and then leave them. The rotting vegetation produces heat, which provides the warmth needed for incubation. Photo by D. Cowell. And each nest type is intimately linked to that bird’s ecology and life history (we’ll return to “life history” later on, but essentially it encompasses the patterns of growth, development, reproduction, and death each species exhibits). If passing your genes on to the next generation is the most important task in an organism’s lifetime To-do list, the nest sits atop the list of essential accessories for most birds. I think it’s easy to take nests for granted. Ultimately, a nest is just a vessel for holding the eggs and nestlings once they hatch. It’s like Nature’s Easter basket; nobody cares about the basket, they just care about the delicious goodies in the basket. But, depending on the species, nests play an important role in hiding, protecting, insulating, and simply retaining a bird’s most precious possessions. As with almost all other facets of bird biology, evolution has shaped every aspect of nest ecology for birds as well; nest placement, materials used for construction, shape, design, etc. Because all of these components of nesting ecology are the result of selection on behavior, the nest can be considered an extension of the bird’s phenotype (quick primer: the phenotype is the physical representation of the genotype). Richard Dawkins, the famous evolutionary biologist, coined the term “the extended phenotype” (and wrote a book titled the same) to help explain how some things made by organisms, such as bird nests, spider webs, and beaver damns, are the product of the same evolutionary forces that produced bird wings, spider silk, or beaver tails. To help illustrate the importance of nests, we’ll take a little tour of different nest types to explore how a species’ nest impacts other components of its life. We’ll start with a nest that is likely familiar to many readers; the nest of the American robin. As illustrated in this time-lapse video, robins typically begin with some loose grass and small twigs, and then build it up using mud as a bonding agent. The inside of the cup is lined with soft, dry grass, and the female uses her body to mold the nest cup into a perfect robin-sized shape. Some robins begin nesting very early in the spring when nighttime temperatures can drop below freezing. These cold temperatures can be lethal to partially developed eggs or nestlings, and the female has to ensure that the occupants of the nest don’t get too cold. It’s possible that the mud used in the nest acts as a form of insulation. Nests vary considerably in the amount of mud that gets incorporated into the structure, and it’s conceivable that some of this variation is linked to expected air temperatures; nests with more mud should be better insulated from very cold temperatures (as well as very hot temperatures). Robins also prefer to place their nests somewhere that is protected on at least two or three sides. The nest is usually built on a platform of some sort—a thick tree branch, a rock outcrop, on the light fixture above your front door- that also has cover above it. This can be in the form of leaves or other thick vegetation, some natural rock or earthen structure, or the eaves of a house or shed. The robin’s nest-location preferences are driven by two principle factors; protection from the elements, and protection from predators. And despite the importance of protection from the elements, nest-predation is thought to be the number one source of nest failure for most avian species. So, in addition to placing the nest in a spot where it is protected from inclement weather and from the searching eyes of predators, robins (and, in fact, many open-cup nest species) have relatively short incubation periods (the time over which they incubate the eggs) and relatively rapid developmental periods (the time over which a newly hatched robin develops into a fledgling that can leave the nest). For a robin, the incubation period lasts about 10-12 days, and the nestling period 10-16 days. Therefore, in the span of 20-28 days, a robin transitions from a just fertilized egg into a fledgling bird capable of short flights. That is an extraordinary feat for a complex, vertebrate organism. How and why does this critical period of growth and development happen so fast? The “how” question is complicated, and involves rapid rates of tissue differentiation and growth, and frankly is outside my realm of expertise. The “why” question is most likely tied back to the bane of the nesting bird; nest predators. If nests are Nature’s Easter baskets, the woods, meadows, and even suburban backyards are teeming with chocolate-crazed children looking to ravage the contents of those Easter baskets. Only, these children come with scales, fur, and feathers. In fact, the list of animals that will depredate (eat) the contents of a bird’s nest is staggeringly long. There are the classical nest-predators, which are animals that routinely take advantage of the seasonal abundance in eggs and nestlings that pop up across the landscape in spring and early summer. These “Usual Suspects” include animals like raccoons, skunks, snakes, crows and jays, hawks, weasels, and owls. And then there are some surprising entrants on the list; many species of squirrel, mouse, and rat, fire and army ants, giant African katydids, and even Bambi. Indeed, based on footage collected from camera-traps, our doe-eyed deer friends seem to quite enjoy the occasional egg snack. Many open-cup nesting species have altricial offspring, which means the baby birds are featherless, blind, and generally helpless upon hatching. As such, they are stuck in the nest for the duration of time they exist in the egg and nestling forms. Only when they have reached the fledgling stage are they capable of moving around under their own power. For nest predators, this means that if they can find a nest during the egg or nestling stage, they have a nice source of protein and fat nicely contained in one convenient (and immobile) package. To find a nest full of juicy nestlings, some predators cue in on the behavior of the parent birds and watch to see where they go. This tactic is most effective during the nestling period when the adults are forced to make frequent trips to the nest to feed their always-hungry offspring. The back and forth movements of the parents provide a target to hone-in on the nest. So how do the parents combat this? One way birds can reduce the predation threat to their nests is to shorten the length of time that the eggs and nestlings are stuck in the nest. Nestlings that can get out of the nest more quickly may be at a lower risk of getting eaten. Alternatively, it may be that the risk doesn’t change much for an individual nestling, but if the nestlings are no longer contained all in one spot (i.e. the nest), the chances of losing all the offspring is lower, especially during one predation event. Presumably, this is where the expression “don’t put all of your eggs in one basket” originated from. To illustrate this dynamic, let’s do some simple math. For the sake of this example, we’ll assume that robins experience a constant level of nest-predation risk over the duration of the egg incubation and nestling periods. If that rate of predation is 0.02 (or 2%) per day, the nest has a 0.56 (or 56%) chance of being eaten over a 28-day period. So that’s a greater than 50% chance of nest failure. If the duration in the nest changes from 28 to 20 days, that value drops to 0.40, or a 40% chance of being eaten. Now, instead of a 56% chance of being eaten (and a 44% chance of surviving), the nest has a 60% chance of surviving to the fledging stage. On an evolutionary scale, that’s a huge difference. Even if we shrink that difference and go with fledging in 24 days, that changes the odds from a greater than even chance of being eaten, to greater than even chance of surviving (52% chance of surviving). Therefore, if species that are generally under high levels of nest-predation pressure can develop more quickly and get out of the nest at a younger age, they may be able to avoid some of that predation and change the odds in their favor. Sounds like a good strategy to me. However, there are almost certainly limitations on how rapidly a bird can develop. Some of these limitations are imposed at a high level of organization—what are called phylogenetic constraints—and are very difficult for evolution to get around. An extreme example of phylogenetic constraint is the evolution of flight in whales. It ain’t gonna happen. But for birds and speed of development, there are limits set by rates of mitosis, cellular differentiation, mitochondrial efficiency, etc., as well as the complexity of building and organizing various tissues and organs. You cannot make an ostrich in a day, or even a week. Another type of limitation comes in the form of developmental and/or life-history costs, and these costs are something I’ve been interested in for the past few years. But we’ll get to those costs in a minute. This raises the obvious question: if you want to avoid losing your eggs/nestlings to hungry predators, but don’t want to deal with these mystery costs associated with rapid development, what’s a nesting bird to do? Some species place their nests in locations that nest predators don’t want to tread. Black-chinned hummingbirds in Arizona like to place their nests near the nests of Cooper’s hawks and northern goshawks because the hawks pose no threat to the hummingbirds (the hummingbirds are too small for the hawks to bother with), but they are threats to the hummingbirds’ nest predators; namely jays. Up in the Arctic, snow geese will sometimes nest in the shadow of a snowy owl nest, and the owls are thought to protect the geese from mammalian nest-predators. Another strategy is to make your nest hard to access, and lots of birds do this by nesting inside cavities. There are some species, like woodpeckers, that excavate their own cavities inside trees (or cacti, in the case of the gilded flicker), and others, like bluebirds, owls, and tree swallows, that take advantage of old woodpecker holes, other natural cavities, and nest-boxes. By nesting inside these structures, cavity-nesters typically experience reduced rates of predation. One sure sign that cavity-nesters are under lower predation pressure comes from the nestlings themselves; they make an unholy racket from inside the nest hole. I’ve found numerous woodpecker nests by following the sounds of nestlings bellowing for food. If a nestling in an open-cup nest made even a fraction of that amount of noise it would be snapped up in an instant by any hungry predator within earshot. In addition to building up their vocal cords, cavity-nesters have the luxury of not having to rush their egg or nestling periods. The eastern bluebird, for example, is a relative of the robin (they’re both in the Turdidae family) and builds its nests in pre-made cavities, including bluebird boxes. The egg incubation period lasts for 11-19 days and the nestling period for between 17 and 21 days. Not only are these longer than the corresponding periods for the robin, but the bluebird is much smaller than the robin—about 30 grams in weight compared the robin’s much more substantial 80 grams—and smaller birds typically develop more rapidly than larger birds when comparing within the same family. What does this more relaxed developmental schedule mean for the cavity nesters? For one, when they do emerge from the nest hole, they are usually much more developmentally advanced than open-cup fledglings. Many of the cavity-nesters can fly reasonably well as soon as they leave the nest, which is critically important for avoiding predators. I’ve talked about the role of predators on nest-survival, but predators are also the presumed number one cause of death in fledgling birds as well. The picture is looking rather rosy for cavity nesters, I would say. They are better protected in the nest, and then better able to avoid predators out of the nest. If I were a robin, I’d start thinking about looking for nest-sites inside tree holes. But there’s more to the story, and this is where the mystery costs of rapid growth and development come in.
Notice to the reader: the rest of this paragraph delves into some complex conceptual ideas and theories about growth and development. If you find your mind beginning to wander, feel free to jump to the next paragraph. Research over the past few decades has shown that when individuals undergo accelerated rates of growth (relative to the norm for that species), they often suffer from a variety of physiological, morphological, and cognitive issues, and ultimately shortened lifespans. These problems have been linked to an excess of glucocorticoid hormones (such as cortisol and corticosterone; the “stress hormones”), as well as high levels of free radicals, which can damage an organism’s cellular machinery and even its DNA. When we look across species, however, it has been harder to identify what costs, if any, are associated with more rapid rates of growth and development. We do tend to see a pattern for species with more rapid rates of growth and development to have shorter lives, and there is some indication that this may be linked to elevated levels of free radicals produced during the rapid growth periods. Some of my own work indicates that there may be another cost as well; a loss of developmental flexibility. Work done in collaboration with colleagues at the University of Illinois examining rates of growth and development across a range of bird species suggests that those species with faster growth experience a reduction in developmental flexibility compared to species with longer periods of growth. This flexibility is important because it allows individuals to adjust their development to fit the prevailing conditions, especially the availability of food. Individuals with more rigid developmental trajectories may be unable to adjust to changing conditions, and could be more likely to die (which is what I found in similar work in zebra finches). Now things really look bad for those open-cup nesters! They’re more likely to die in the nest. They’re more likely to die out of the nest as fledgers. And then they’re more likely to suffer from a litany of ailments that ultimately result in death at an early age. Where do I sign up? But the reality is that there’s something called a life-history tradeoff, which means, more or less, that some species live a fast-paced lifestyle, and other live a slow-paced lifestyle. Species that live life in the fast lane grow rapidly, typically have lots of offspring, and die young. The idea is that the relative parental investment is pretty low for a given individual offspring. And this is where a lot of the open-cup nesting species fall; they can make up for the high rates of mortality by producing more offspring. Meanwhile, the species in the slow lane generally live longer, produce fewer offspring per year, but invest more into each individual. Within the bird world, the species on the extreme slow end of the spectrum are seabirds like albatrosses, which can live to be 70 years old, often don’t reproduce until they’re 10+ years old, and raise a single chick over an 8-10 month period. Cavity nesters like bluebirds don’t exhibit that kind of life-history, but they do invest more energy and resources into a given clutch than robins do, simply by way of incubating the eggs for longer, and provisioning the nestlings for longer. I’ve only scratched the surface in this exploration of avian nesting ecology, but I think I’ll leave it there for now. Hopefully readers will have the opportunity to see some of this nesting ecology in action for themselves over the coming months. And on a related note, happy belated Mother’s Day to all the mom’s out there, especially my mom! Here’s the link to my Mother’s Day post from last year. Next post: TBD Subscribe to the Newsletter! If you would like to get notifications about when new posts are up and other tidbits related to the blog, sign up for the View Out the Door twice-monthly newsletter. Just email viewoutthedoor “at” gmail “dot” com with the subject header SUBSCRIBE. |
About the author:Loren grew up in the wilds of Boston, Massachusetts, and honed his natural history skills in the urban backyard. He attended Cornell University for his undergraduate degree in Natural Resources, and received his PhD in Ecology from the University of California, Santa Barbara. He has traveled extensively, and in the past few years has developed an affliction for wildlife photography. Archives:
|